My longstanding research interests are to understand how the brain encodes, discriminates, and relays our complex contextual cues for adaptive calibration of defensive and motivated behaviors.
Indeed, failures to properly scale one’s behavior to situation-specific circumstances is a hallmark of many psychiatric illnesses, whether that is for the anxious individual struggling to minimize their anxiety and fear outside the clinic, or the binge-eating patient who finds it extremely difficult to limit their consumption, no matter the context.
I believe the identification of cells and circuits that mediate commonly shared mechanisms of contextual control is a promising path forward in the development of novel behavioral and pharmacological therapies for minimizing relapse of unwanted behaviors.
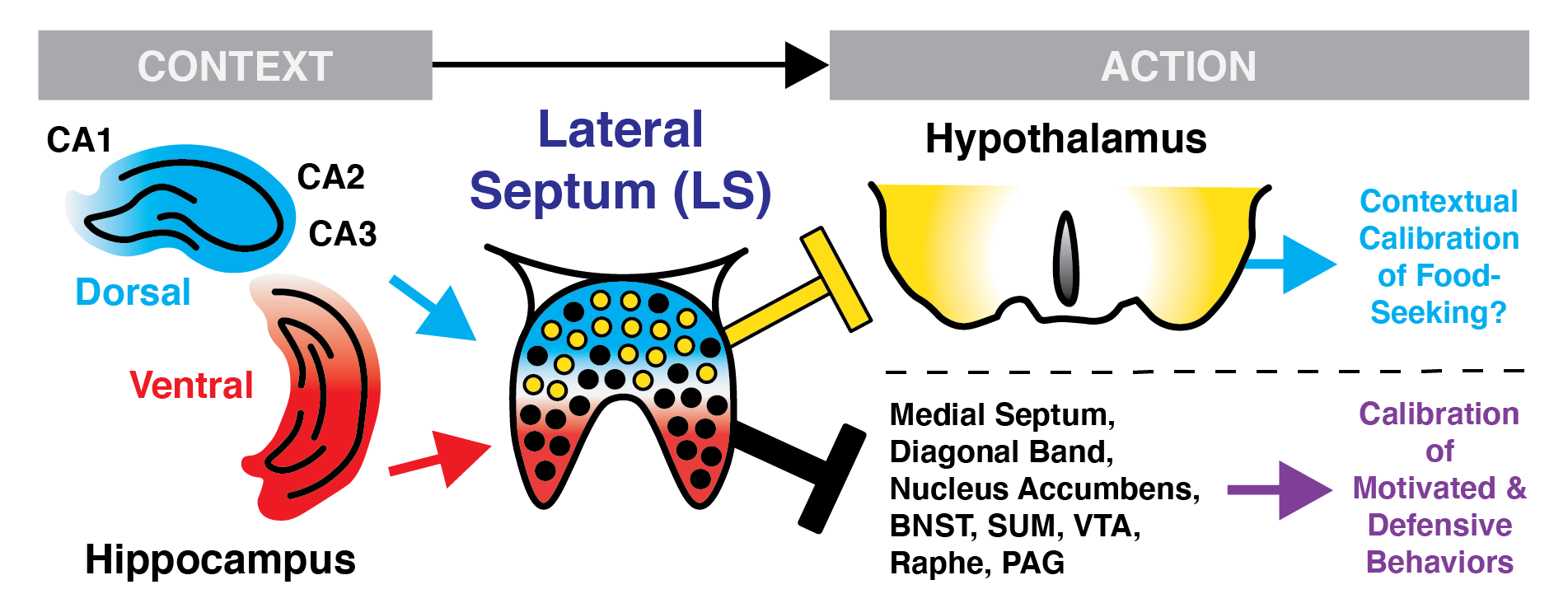
Of course, decades of work have revealed that the hippocampus (HPC) is fundamental to the acquisition and expression of context-dependent memory, including of fearful or rewarding (food/social/drug) experiences. This is theorized to occur through its capacity to transform conjunctive sensory signals into sparse, unique, and linked patterns of activity (“engrams”) that are indexed and reactivated and deployed for precise memory recall and (re)consolidation via its diverse brain-wide network.
Unlike the majority of the HPC’s extended network to the subcortex, the evolutionarily conserved lateral septum (LS) receives broad input from across subregions of both the dorsal and ventral HPC—in turn, the broad inhibitory network of the LS provides considerable GABAergic input onto various regions of the hypothalamus and subcortical circuits.
Perhaps then it is not surprising that there is now considerable interest in the LS as a critical nexus for contextual regulation of defensive and motivated behaviors.
However, the LS is comprised of multiple layers and substructures, whose borders, cell-types, inter- and extra-LS connections, sexual dimorphisms, and functions of each remain poorly defined or explored.
As such, deconstruction of the functional organization of the LS harbors untapped scientific and therapeutic potential, and my lab will focus on the LS and its extended HPC-subcortical network using modern molecular and circuit tools in preclinical studies of context-dependent aversive (defensive fear/freezing) and motivated (feeding/social) behaviors to test how learning shapes its signals across different “contexts”.

My Expertise (& Tools My Future Lab Will Use):
Behavioral Tracking (Noldus/SLEAP/DeepLabCut) — Biosafety (Lvl 1/2) — Cell Culture/Mini-/Maxi-Prep/Lentivirus Preparation— Chemogenetics/DREADDs — Combinatorial Tracing of Circuits (AAV/Rabies/CTb) — ELISA — Engram Labeling/Reactivation (Fos-tTA/TRAP2/CAL-Light) — Ex Vivo Slice Electrophysiology — Fiber Photometry — Fluorescent/Chromogenic Immunohistochemistry (IHC) — Grantsmanship — Histology/Cryosectioning/Tissue Dissection/Fixation — In Situ Hybridization (F/ISH/RNAScope) —In Vivo 1-P Calcium Imaging — In Vivo Electrophysiology (Single Unit) — Intracranial Stereotactic Surgery — Intracranial/Systemic Pharmacology — Light/Fluorescent/Confocal Microscopy — Mentoring/Teaching/Pedagogy/Public Speaking — Mouse Colony Management/Genotyping/PCR — Noldus/Ethovision/Med-PC — Optogenetics/Fiber Production/Lasers — Pavlovian Fear Conditioning/Extinction/Relapse — Programming (R/RStudio/Matlab) — Responsible Conduct of Research (RCR) — Rodent Handling/Animal Husbandry (Mouse/Rat/Hamster) — Statistics — Sterilization/Autoclaving — Tests for Anxiogenesis/Locomotion (OF/EPM) — Tests for Feeding/Food-Seeking (Context-Induced Overconsumption/NSF) — Tests for Social Behavior (Social Defeat/Conditioned Defeat/Social Discrimination) — Tissue Clearing/CLARITY — Tissue Quantification/ImageJ — Transcriptomics/Single-Nucleus RNA-Sequencing (snRNAseq/10X) — Valence Assays/Real-Time Place Preference (RTPP) — Western Blotting
My Published Works (Click for Links)
My Profiles: Twitter/X | Bluesky | Mastodon | LinkedIn | Harvard Catalyst | Web of Science | Loop | ORCiD | ResearchGate | NeuroTree
(Disclaimer: I Do Not Speak For Any Employer Or Funding Agency; All Opinions My Own)



